Rethinking Nuclear Medicine Safety in a Fast-Changing World
Nuclear medicine has become a cornerstone of modern healthcare, enabling earlier diagnoses, more precise treatments and better monitoring of complex diseases such as cancer and cardiovascular disorders. But behind every radiopharmaceutical injection, every PET-CT scan and every intervention in a cath lab lies an invisible risk: exposure to ionising radiation for healthcare professionals, patients and even the surrounding environment.
While regulations and international guidelines have tightened over the last decades, radiation protection practices still vary widely from one country to another, and even from one hospital to the next. Many facilities continue to operate with ageing equipment, improvised shielding or suboptimal workflows that can increase unnecessary exposure.
In this context, a French company has quietly become a central player in transforming how safety is conceived, designed and implemented in nuclear medicine departments worldwide. Founded in 1970 near Nantes, on the Atlantic coast, Lemer Pax has evolved from a lead-working specialist into a global innovation leader in radiation protection – with a mission that can be summed up in two words: “Protecting Life”.
From Lead Foundry to Global Reference in Radiological Protection
The origins of Lemer Pax lie in a highly specialised expertise: working and shaping lead for shielding purposes. Over time, as nuclear technologies developed in medicine, industry and research, the company leveraged this know-how to address a growing need: protecting people from ionising radiation without compromising performance or precision.
Over more than five decades, the company has steadily broadened its scope. Today, it provides solutions in four major domains:
- Medical – nuclear medicine, radiology and interventional cardiology
- Research – physics laboratories, radiochemistry, fundamental science
- Industry – non-destructive testing, energy, high-tech manufacturing
- Civil nuclear – power plants, waste management, decommissioning
Its evolution has been driven by a dual dynamic: on one hand, tightening regulatory requirements and, on the other, a clear expectation from clinicians and operators to work in better protected, more ergonomic and more efficient environments. Rather than simply supplying shielding components, the company now designs integrated, turnkey solutions that combine materials science, mechanical engineering, automation and human factors.
How Lemer Pax Is Transforming Safety in Nuclear Medicine Departments
The most visible impact of the company’s work is in hospitals and clinics, particularly in nuclear medicine and interventional cardiology. These are environments where both chronic low-dose exposure and potential accidental overexposures must be taken seriously.
In nuclear medicine, three challenges stand out:
- Minimising the dose received by staff during preparation and administration of radiopharmaceuticals
- Securing the entire workflow, from delivery and storage to waste handling
- Maintaining or improving exam throughput and diagnostic quality while enhancing safety
Over the years, Lemer Pax has built a comprehensive portfolio of medical devices and shielding systems that address these pain points in a systemic way.
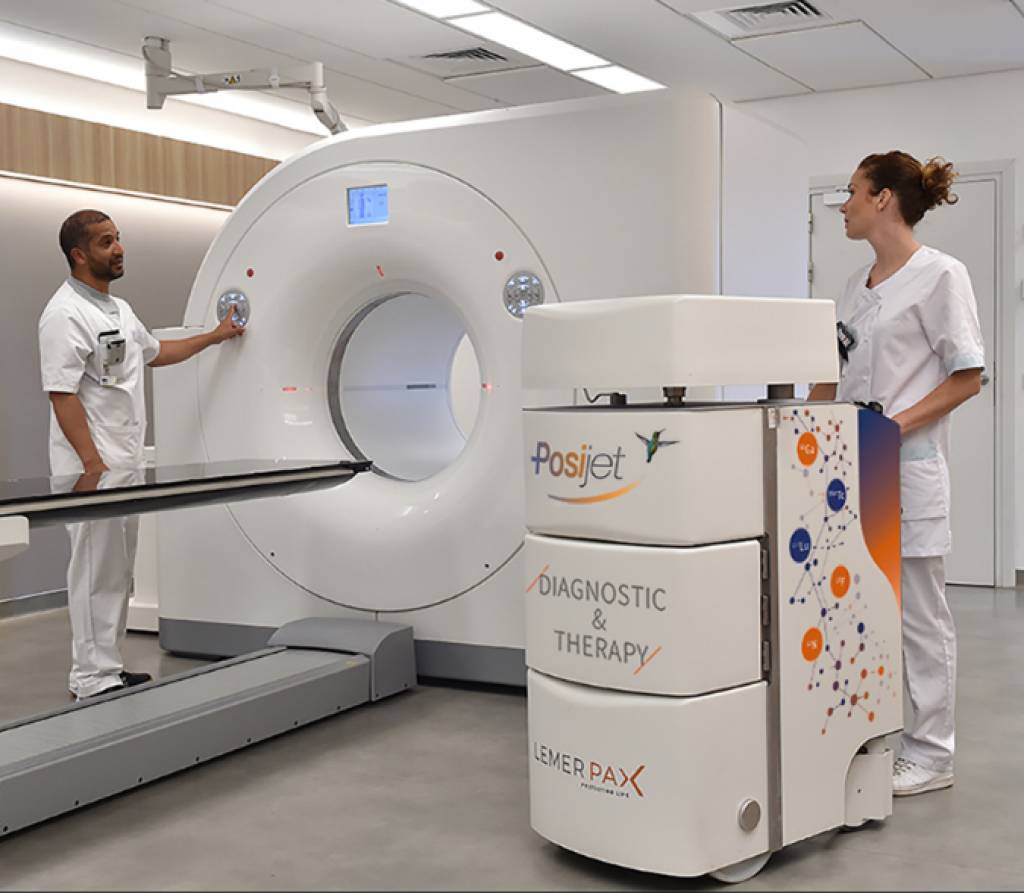
Shielded Injectors: Protecting Staff Without Delaying Patient Care
One of the most emblematic innovations in this field is the shielded injector for radiopharmaceuticals. Traditionally, technologists and nurses manually handle syringes containing radioactive tracers such as FDG or technetium-based compounds. Even with handheld syringe shields, repeated exposure throughout the day adds up to significant cumulative doses.
Shielded injectors enclose the radiopharmaceutical in a heavily protected housing and automate or semi-automate the injection process. The benefits are multiple:
- Reduced occupational exposure – the operator stands at a greater distance and behind robust shielding during injection
- Reproducible dosing – automated systems help secure accurate and consistent injected activities
- Workflow optimisation – better organisation of injection sequences can increase patient throughput
- Improved ergonomics – less time spent in direct contact with radioactive materials, reduced physical strain
By integrating these injectors into existing imaging workflows, hospitals can align their practices with the ALARA principle (“As Low As Reasonably Achievable”) without sacrificing efficiency or patient experience.
Preparation Enclosures: Safe, Clean and Compliant Radiopharmacy
Before any injection takes place, radiopharmaceuticals must be prepared and dispensed, often within on-site radiopharmacies or hot labs. This stage carries a double risk: radiological and biological. Contamination, spills or handling errors can expose staff and compromise regulatory compliance.
To address this, the company designs and manufactures shielded preparation enclosures that function as controlled, clean and secure micro-environments for radiopharmaceutical handling. These enclosures often integrate:
- High-performance shielding in multiple directions to limit ambient dose rates
- Airflow and filtration systems for aseptic preparation in line with pharmaceutical standards
- Ergonomic workstations with optimised height, visibility and access
- Integrated storage, waste and monitoring solutions for safe containment
The goal is to create a radiopharmacy where staff can concentrate on the precision of their manipulations rather than on the fear of exposure or contamination incidents. This aligns with the increasing demand for GMP-level (Good Manufacturing Practice) environments in hospital-based radiopharmacies, especially as theranostic agents and short-lived isotopes become more widely used.
Mobile Shielding: Flexible Protection in Dynamic Clinical Spaces
Another critical area is the examination room itself. Physicians, technologists and nurses must often remain close to patients who are temporarily radioactive following injection. In hybrid operating rooms or interventional cardiology suites, operators may spend long hours near fluoroscopy systems and injected patients.
To mitigate this, Lemer Pax offers mobile shielding devices such as movable screens and barriers. These paravents are designed to be:
- Highly manoeuvrable to adapt to tight or changing layouts
- Precisely shielded with materials and thicknesses calculated for specific energy ranges
- Transparent or semi-transparent in key areas to maintain visual contact with the patient
- Compatible with infection control through easy-clean surfaces and robust finishes
In interventional cardiology, these systems complement ceiling-suspended shields and personal protection such as lead aprons, aiming to reduce both whole-body dose and specific organ exposure (particularly to the head and neck of operators).
A Global Footprint in Industry, Research and Civil Nuclear
While medical applications may be the most visible to the general public, a significant share of the company’s activities lies beyond hospital walls. In industry and research, ionising radiation is used for material testing, imaging, sterilisation and fundamental physics.
Here, Lemer Pax develops and supplies:
- High-technology viewports that allow operators to observe processes inside shielded cells without compromising safety
- Transport containers (“casks”) for moving radioactive sources or materials between facilities or within complex sites
- Manipulation cells and hot cells for handling highly active materials with remote tools
These solutions are tailored to the constraints of each sector, whether it is the robustness and reliability required in nuclear power plants or the ultra-high precision demanded by fundamental research laboratories.
The company’s know-how has enabled it to contribute to ambitious international scientific endeavours, including projects related to dark matter research. In such experiments, background radiation must be controlled at unprecedented levels, making advanced shielding an integral part of the experimental apparatus rather than an afterthought.
Innovation as a Core Strategy: 80+ Patents and Counting
One of the reasons why Lemer Pax has become a global reference in radiation protection is its systematic investment in research and development. With an impressive portfolio of more than 80 patents filed internationally, the company has turned innovation into a strategic asset rather than a marketing slogan.
This innovation is not limited to incremental improvements in shielding thickness or geometry. It extends to materials, manufacturing processes, system integration and user experience. A particularly significant shift has been the move towards more ecological and sustainable materials.
Traditionally, radiation protection has relied heavily on lead because of its high density and attenuation properties. However, lead presents environmental and health challenges, particularly in manufacturing and end-of-life management. In response, the company has developed high-performance lead-free alloys that offer comparable, and sometimes superior, protective capabilities without the same ecological footprint.
These new materials are part of a broader eco-design approach that looks at the entire life cycle of products: sourcing, fabrication, transport, use and recycling. In a sector often perceived as heavy and conservative, such an approach represents a meaningful leap towards more responsible industrial practices.
Engineering, R&D and the New “Monarch” Headquarters
With more than 140 employees, the company relies on a multidisciplinary mix of engineers, technicians, researchers and field experts. This team supports an agile R&D process that can respond rapidly to emerging needs, whether they stem from regulatory changes, new isotopes or novel clinical procedures.
In 2024, the company marked a major milestone by moving into a new industrial site known as “Le Monarch” in Carquefou, near Nantes. This modern headquarters consolidates design offices, production workshops and administrative departments under one roof.
Bringing these activities together is more than a logistical convenience. It enhances collaboration between design and manufacturing, shortens development cycles and allows prototypes to be quickly tested, adjusted and industrialised. For clients, this translates into more reliable delivery times and a closer alignment between their practical needs and the final products.
Co-Creation with Clinicians and Academic Experts
Another key aspect of the company’s innovation model is its close collaboration with healthcare professionals and academic researchers. Rather than designing products in isolation, engineers work alongside nuclear medicine physicians, radiopharmacists, interventional cardiologists and technologists.
This co-creation process typically involves:
- On-site observations to understand real workflows and constraints
- Iterative prototyping with direct feedback from end-users
- Joint evaluation of ergonomics, safety and clinical performance
- Adaptation to local regulatory requirements and cultural practices
The result is equipment that not only meets theoretical safety standards but genuinely integrates into the daily routines of departments. Enhanced comfort for operators, intuitive controls and compatibility with existing infrastructure all help ensure that safety features are actually used as intended, rather than bypassed in the rush of clinical activity.
Global Reach and Regulatory Excellence
Radiation protection is one of the most regulated areas in healthcare and industry. Products must comply with national and international standards that can vary from one region to another. Certification processes are demanding and failure to comply can have serious consequences.
Over the years, the company has built a robust quality management system aligned with some of the strictest norms in the field. This gives hospitals, laboratories and industrial clients the assurance that the equipment they use not only performs technically but also meets or exceeds legal and regulatory expectations.
A significant portion of the company’s turnover is generated outside France, with exports reaching all five continents. This international presence has required a deep understanding of diverse regulatory frameworks, from European directives to North American and Asian standards. It has also led to the creation of a network of local partners who provide installation, training and after-sales support, ensuring continuity of service throughout the product life cycle.
A Responsible Industrial Actor in a Sensitive Sector
Operating in the nuclear and medical fields comes with a heightened responsibility towards society. Beyond product performance, stakeholders expect ethical behaviour, transparency and environmental stewardship.
The company has integrated these expectations into a broader corporate social responsibility (CSR) strategy. This includes:
- Short supply chains whenever possible, to reduce transport-related emissions and support local economies
- Eco-design principles that favour durable, repairable and, where possible, recyclable systems
- Continuous training for staff on safety, quality and environmental issues
- Engagement with the scientific community through participation in major research projects
In a context where public perception of nuclear technologies is sometimes ambivalent, such commitments help reinforce the legitimacy of medical and industrial uses of radiation by demonstrating that they can be developed responsibly.
Raising the Bar for Radiological Protection Standards
As nuclear medicine continues to expand – with new tracers, theranostic approaches and hybrid imaging techniques – the question of safety will remain central. Staff shortages, ageing infrastructure and increasing patient volumes put pressure on departments and heighten the risk of shortcuts in protection measures.
By combining advanced engineering, innovative materials and user-centric design, Lemer Pax has contributed to turning radiological protection into a true standard of excellence rather than a mere regulatory checkbox. Its solutions show that it is possible to:
- Significantly reduce occupational exposure without sacrificing workflow efficiency
- Integrate robust shielding into ergonomically optimised workspaces
- Design environmentally responsible protection systems in a historically heavy industry
- Support cutting-edge scientific research while managing radiological and environmental risks
For healthcare institutions, research laboratories and industrial operators worldwide, the message is clear: safety in nuclear medicine and radiation-related activities is no longer just about complying with minimum standards. It is about adopting a comprehensive, forward-looking approach that protects professionals, patients and the environment – and helps ensure that the benefits of nuclear technologies can be harnessed with confidence for decades to come.